42 Draw The Lewis Structure For N2H2
Web the lewis structure of n2h2 consists of two nitrogen (n) atoms at the center which are bonded to two atoms of hydrogen (h), one on each side. To draw the n2h2 lewis structure, the valence electrons of each atom are represented by dots or lines, while the bonding pairs are depicted by lines between the atoms. Hch2 367 carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized. This compound is most commonly known as diazene or diimide. Select the center atom (h is always outside).
Draw the lewis structures of n2h4, n2h2, and n2. Neutral compounds with a bite sized video explanation from jules bruno start learning comments (2) video transcript question 7 textbook question Calculate the total number of valence electrons. Dinitrogen dihydride has the chemical formula of n2h2. This compound is most commonly known as diazene or diimide.
Web part a draw the lewis structure for n2h2. Draw the lewis structures of n2h4, n2h2, and n2. Draw the lewis structures of n_2h_4, n_2h_2, and n_2. Here, the given molecule is n2h2 (or dinitrogen dihydride). To draw the n2h2 lewis structure, the valence electrons of each atom are represented by dots or lines, while the bonding pairs are depicted by lines between the atoms.
N2H2 Lewis Structure How to Draw the Lewis Structure for Dinitrogen
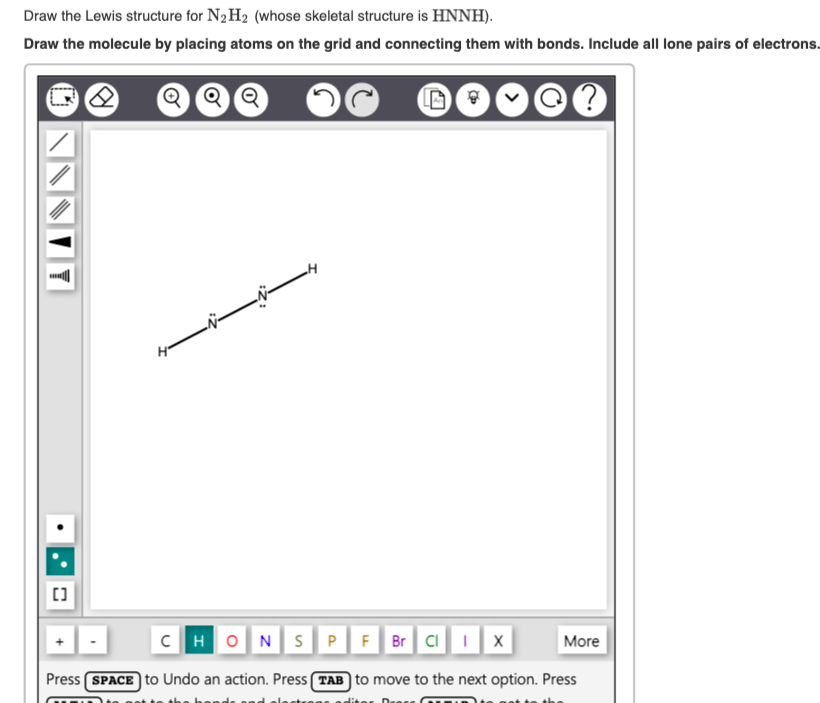
The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Draw the molecule by placing atoms on the grid and connecting them with bonds..
N2h2 Lewis Structure Molecular Geometry Draw Easy
Draw lewis structures for the hydrazine molecule ( n2h4), the nitrogen molecule (n2), and the diazene molecule (n2h2), and then answer the questions that follow. Calculate the total number of valence electrons. For the n2h2.
How to Draw the Lewis Dot Structure for N2H2 Diazene YouTube
#1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary #4 minimize formal charges by converting lone pairs of the.
N2H2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
For the n2h2 structure use the periodic table to find the total number of valence. • draw one structure per sketcher box, and separate added sketcher boxes with the + sign. Key takeaways understanding lewis.
N2H2 Lewis structure, Molecular Geometry, Hybridization, Bond Angle and
This structure helps us understand the bonding and electron distribution in the molecule. Web today in this video we help you determine the lewis structure of dinitrogen dihydride. Carbons 2 and 3 are sp3, carbons.
N2H2 Lewis Structure (Dinitrogen Dihydride) How to find out
Web drawing lewis structures for molecules with one central atom: To draw the n2h2 lewis structure, the valence electrons of each atom are represented by dots or lines, while the bonding pairs are depicted by.
Draw the Lewis structures of N2H4, N2H2, and N2 YouTube
Web the lewis structure of n2h2 shows that it consists of two nitrogen atoms bonded together with a double bond, each nitrogen atom also bonded to two hydrogen atoms. Include all lone pairs of electrons.
N2h2 Lewis Structure Molecular Geometry Draw Easy
Draw the lewis structures of n2h4, n2h2, and. Web the lewis structure of n2h2 shows that it consists of two nitrogen atoms bonded together with a double bond, each nitrogen atom also bonded to two.
Lewis Dot Structure For N2h2 Draw Easy
Here, the given molecule is n2h2 (or dinitrogen dihydride). Web chemistry questions and answers. Web determine the lewis dot structure for the diazene molecule, n 2 h 2. Thus far, we have discussed the various.
Solved Draw the Lewis structure for N2H2 (whose skeletal
Draw the molecules by placing atoms on the grid and connecting them with bonds. Web by using the following steps, you can easily draw the lewis structure of n 2 h 2: Draw the lewis.
What is the hybridization on the n atoms? Draw the lewis structures of n2h4, n2h2, and. Web chemistry chemistry questions and answers draw the lewis structures of n2h4, n2h2, and n2. Understand the proper use of the octet rule to predict bonding in simple molecules. • draw one structure per sketcher box, and separate added sketcher boxes with the + sign. Let us find out the lewis structure of dinitrogen dihydride, n2h2. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web n2h2 lewis structure molecular geometry hybridization and mo diagram. A) ccl4 b) n2h2 c) hcn 6) a) draw the molecular orbital diagram of the given compounds. Web part a draw the lewis structure for n2h2. Include all lone pairs of electrons. This compound is most commonly known as diazene or diimide. For the n2h2 structure use the periodic table to find the total number of valence. This structure helps us understand the bonding and electron distribution in the molecule. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary #4 minimize formal charges by converting lone pairs of the atoms #5 repeat step 4 if necessary, until all charges are minimized