21 Draw The Products Formed From The Ester Hydrolysis Reaction Shown
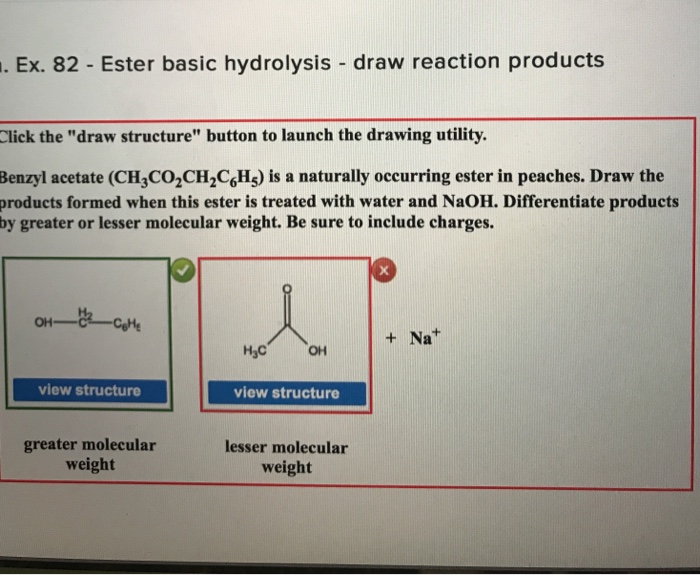
Web because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification from the latin sapon, meaning “soap,” and facere,. You'll get a detailed solution from a subject matter expert that. So if we increase the concentration of water, that would shift the equilibrium back to the. Web identify the products of a basic hydrolysis of an ester. Web when a base (such as sodium hydroxide [naoh] or potassium hydroxide [koh]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol.
Esters are neutral compounds, unlike the acids from which they are formed. Web in this video, we're talking about the reverse reaction; We're going to talk about ester hydrolysis. We’ll focus on hydrolysis, saponification, aminolysis, and polytransesterification. An ester is a derivative of a carboxylic acid.
Esters are neutral compounds, unlike the acids from which they are formed. Web this is the usual way of hydrolyzing esters. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution. We're going to talk about ester hydrolysis. And maybe a more general way of looking at this reaction is as an ester hydrolysis.
Draw The Products Formed From The Ester Hydrolysis Reaction Shown
In typical reactions, the alkoxy (or′) group. Web identify the products of a basic hydrolysis of an ester. Part a draw the products formed from the ester hydrolysis reaction shown. In typical reactions, the alkoxy.
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps
Web the reaction is catalyzed by dilute acid, and so the ester is heated under reflux with a dilute acid like dilute hydrochloric acid or dilute sulfuric acid. Web when a base (such as sodium.
Solved . Ex. 82 Ester basic hydrolysis draw reaction
Web identify the products of a basic hydrolysis of an ester. We have seen that all the steps in the fischer esterification are reversible. That is exactly what happens when esters are hydrolysed by water.
Draw The Products Formed From The Ester Hydrolysis Reaction Shown
We're going to talk about ester hydrolysis. Web in this video, we're talking about the reverse reaction; Web the reaction is catalyzed by dilute acid, and so the ester is heated under reflux with a.
Question Video Identifying the Molecular Formula of the Ester That Is
An ester is a derivative of a carboxylic acid. Web carboxylic acids and their derivatives. We’ll focus on hydrolysis, saponification, aminolysis, and polytransesterification. That is exactly what happens when esters are hydrolysed by water or.
Draw the products formed when each ester is hydrolyze… SolvedLib
Web because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification from the latin sapon, meaning “soap,” and facere,. Web identify the products of a basic.
Simple Hydrolysis Reaction
Web 100% (1 rating) transcribed image text: In typical reactions, the alkoxy (or′) group. You'll get a detailed solution from a subject matter expert that. Web identify the products of a basic hydrolysis of an.
basic ester hydrolysis mechanism
Web when a base (such as sodium hydroxide [naoh] or potassium hydroxide [koh]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Part a draw the products formed from.
Draw The Products Formed From The Ester Hydrolysis Reaction Shown
We're going to talk about ester hydrolysis. There are two advantages of doing this. Web when a base (such as sodium hydroxide [naoh] or potassium hydroxide [koh]) is used to hydrolyze an ester, the products.
Draw The Products Formed From The Ester Hydrolysis Reaction Shown
In typical reactions, the alkoxy (or′) group of an ester is. An ester is a derivative of a carboxylic acid. In typical reactions, the alkoxy (or′) group. So if we increase the concentration of water,.
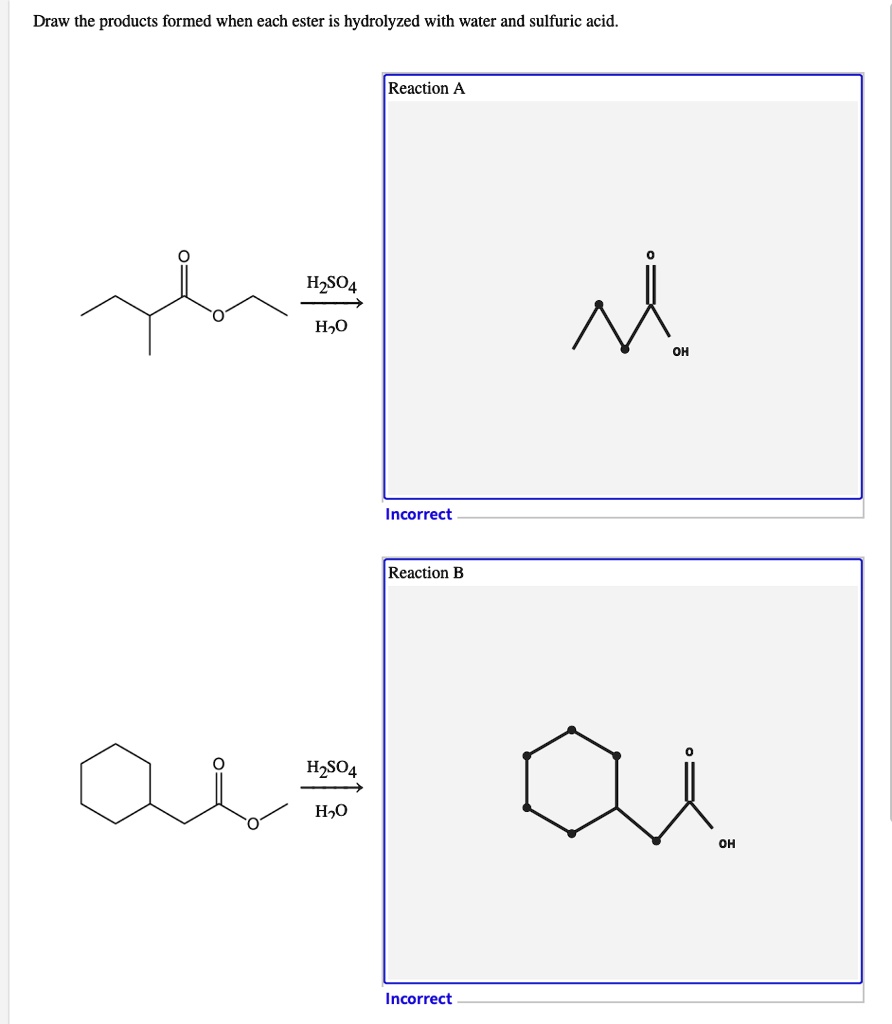
That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. Part a draw the products formed from the ester hydrolysis reaction shown. Web draw the products of the hydrolysis reaction between ester molecule and water this problem has been solved! Web identify the products of a basic hydrolysis of an ester. Web this is the usual way of hydrolyzing esters. Web identify the products of a basic hydrolysis of an ester. Esters are neutral compounds, unlike the acids from which they are formed. Part a draw the products formed from the ester hydrolysis reaction shown. In typical reactions, the alkoxy (or′) group of an ester is. Web when a base (such as sodium hydroxide [naoh] or potassium hydroxide [koh]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Web because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification from the latin sapon, meaning “soap,” and facere,. Web the reaction is catalyzed by dilute acid, and so the ester is heated under reflux with a dilute acid like dilute hydrochloric acid or dilute sulfuric acid. Web technically, hydrolysis is a reaction with water. So if we increase the concentration of water, that would shift the equilibrium back to the. In typical reactions, the alkoxy (or′) group.