49 Surface Tension Drawing
Web surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force acts: Since these intermolecular forces vary depending on the nature of the liquid (e.g. Web “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise surface area”. An overview of intermolecular forces in action as surface tension, viscosity, and capillary action. Web surface tension has the units of force per length, and its action is confined to the free surface.
Gasoline) or solutes in the liquid (e.g. Its si unit is newtons per metre, and its cgs unit is dynes per centimetre. Web properties of fluids surface tension the surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid. Table 1 gives the value of the surface tension for some typical materials. Since these intermolecular forces vary depending on the nature of the liquid (e.g.
Web this effect results from the difference between the potential energy of atomic interactions on the interface between two different fluids and that in their bulks, and thus may be described by an additional potential energy. Table 1 gives the value of the surface tension for some typical materials. Ui = γa, (8.2.1) (8.2.1) u i = γ a, where a a is the interface area, and γ γ is called the surface tension constant. Gasoline) or solutes in the liquid (e.g. If the surface line element is a closed loop
Surface Tension Chemistry LibreTexts
The directed contracting force which attracts the molecules at the surface of a liquid towards the. Web “surface tension is the tension of the surface film of a liquid caused by the attraction of the.
Schematic illustration of standard methods of surface tension
Web surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of a liquid results from an imbalance of intermolecular attractive forces,.
Illustration of the modeling of the surface tension forces with the
The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also.
Surface Tension Stock Illustration Download Image Now iStock
If the surface line element is a closed loop Cover your work surface with paper towels. It is also responsible for the beading up of water droplets on a freshly waxed car because there are.
Surface tension explanation vector illustration diagram Stock Vector
Consider for the sake of simplicity a perfectly flat interface. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension has the dimension of force per unit length, or of.
Explain the surface tension phenomenon with examples.
Web this effect results from the difference between the potential energy of atomic interactions on the interface between two different fluids and that in their bulks, and thus may be described by an additional potential.
Surface Tension Drawing & Painting on Aluminium Jackson's Art Blog
Web surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force acts: Web surface tension is the energy, or.
What is Surface Tension? CTG Technical Blog
Web liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Web surface tension art materials:. The intermolecular forces between molecules in the liquid state vary depending upon.
10 Surface Tension Examples in Daily Life StudiousGuy
Table 1 gives the value of the surface tension for some typical materials. Web explore surface tension and how it varies from one liquid to another. This creates surface tension, which allows for phenomena such.
FileSurface Tension Diagram.svg Wikipedia
Surfactants like detergent), each solution exhibits differing surface tension properties. Web the force per unit length perpendicular to a line drawn in the surface of the liquid is the surface tension. Web • surface tension.
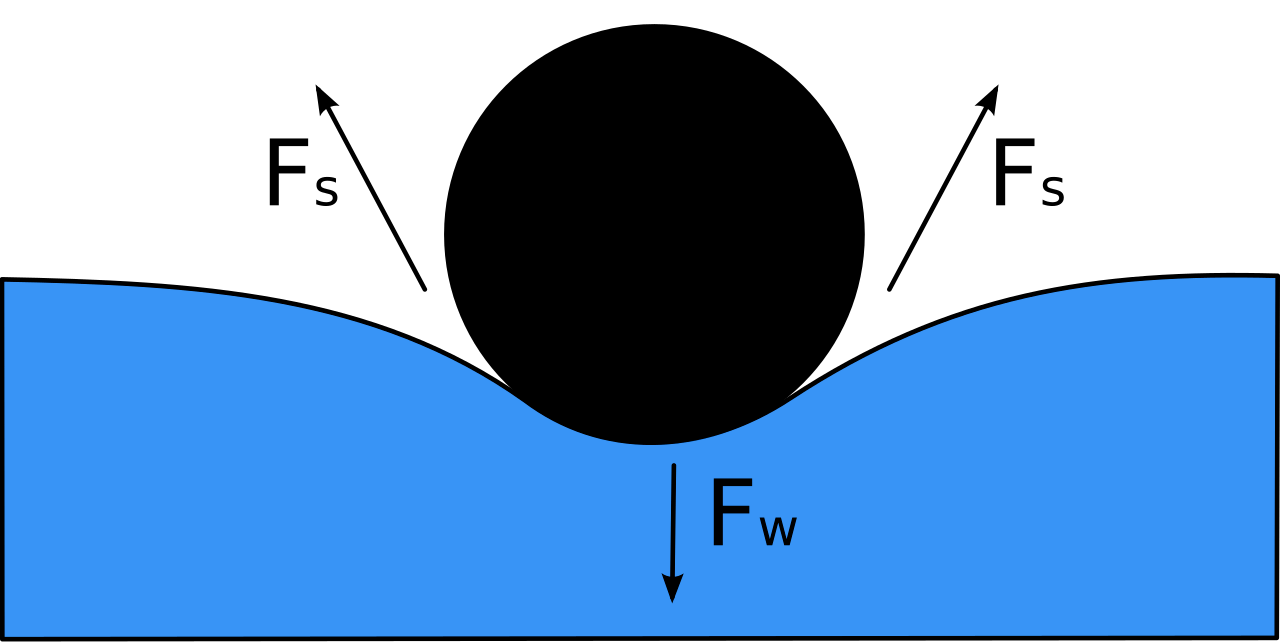
Surface tension has the dimension of force per unit length, or of energy per unit area. This property results from the cohesive forces between molecules at the surface of a liquid, and it causes the surface of a liquid to behave like a stretched rubber membrane. The directed contracting force which attracts the molecules at the surface of a liquid towards the. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: Web surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Web the force per unit length perpendicular to a line drawn in the surface of the liquid is the surface tension. Web this effect results from the difference between the potential energy of atomic interactions on the interface between two different fluids and that in their bulks, and thus may be described by an additional potential energy. Learn about surface tension and compare the surface tensions of different liquids, including water, alcohol, mercury, and soap bubbles. Gasoline) or solutes in the liquid (e.g. Web surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gamma = f / d units of surface tension surface tension is measured in si units of n/m (newton per meter), although the more common unit is the cgs unit dyn/cm (dyne per centimeter). Web the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Web surface tension allows objects that are denser than water, such as the paper clip shown in b in the figure below, to nonetheless float on its surface. This creates surface tension, which allows for phenomena such as water droplets maintaining a round shape and insects walking on water. Table 1 gives the value of the surface tension for some typical materials.